Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Collaborative Laboratories for Advanced Decommissioning Science; Hokkaido University*
JAEA-Review 2021-036, 95 Pages, 2021/12
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Safe, efficient cementation of challenging radioactive wastes using alkali activated materials with high-flowability and high-anion retention capacity" conducted in FY2020. For safe storage and disposal of iron sludge generated from contaminated water treatment, the present study aims to 1) explore alkali activated materials (AAM) with high-flowability and high-anion retention capacity and its recipe, 2) try mock-up manufacture and evaluation for one-tenth the size of real waste and propose the concept of the manufacturing equipment for a real plant, 3) show potential of AAM as the material for the solidification of waste with various physicochemical properties and radioactive nuclide compositions from the result ...
Ouchi, Kazuki; Tsukahara, Takehiko*; Brandt, A.*; Muto, Yuki*; Nabatame, Nozomi*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1789 - 1794, 2021/12
Times Cited Count:1 Percentile:6.35(Chemistry, Analytical)We attempted to scale down a separation process of uranium (U) using the microchip column loaded with anion exchange resin to develop safety and waste-reducing separation technique. The ideal separation performance of U was obtained by the properly design of a microchannel. The concentration of U in seawater as a real-world sample could be quantified with the prepared microchip column. It indicates that the microchip column is sufficiently practical. Compared to separation of U with a general column, the column size was successfully scaled down to  1/5000.
1/5000.
Collaborative Laboratories for Advanced Decommissioning Science; Hokkaido University*
JAEA-Review 2020-054, 72 Pages, 2021/01
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2019. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2019, this report summarizes the research results of the "Safe, efficient cementation of challenging radioactive wastes using alkali activated materials with high-flowability and high-anion retention capacity". The purpose of this study is to find safe, efficient cementation of challenging radioactive wastes using alkali activated materials with high-flowability and high-anion retention capacity, and to propose the concept of a manufacturing apparatus that is established as an actual plant. As a result of study in this year, it was revealed that the K-based alkali activated material has high-flowability and quick curing, and that high-iodine retention capacity is achieved by incorporating silver ions during manufacturing of solidified waste.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:18 Percentile:69.03(Chemistry, Multidisciplinary)Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:4 Percentile:12.17(Chemistry, Analytical)no abstracts in English
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:9.05(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
Ozawa, Mayumi; Fukaya, Hiroyuki; Sato, Makoto; Kamohara, Keiko*; Suyama, Kenya; Tonoike, Kotaro; Oki, Keiichi; Umeda, Miki
Proceedings of 53rd Annual Meeting of Hot Laboratories and Remote Handling Working Group (HOTLAB 2016) (Internet), 9 Pages, 2016/11
Zhao, Y.; Yoshimura, Kimio; Shishitani, Hideyuki*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Koizumi, Satoshi*; Szekely, N.*; Radulescu, A.*; Richter, D.*; Maekawa, Yasunari
Soft Matter, 12(5), p.1567 - 1578, 2016/02
Times Cited Count:27 Percentile:80.24(Chemistry, Physical)Yoshimura, Kimio; Koshikawa, Hiroshi; Yamaki, Tetsuya; Shishitani, Hideyuki*; Yamamoto, Kazuya*; Yamaguchi, Susumu*; Tanaka, Hirohisa*; Maekawa, Yasunari
Journal of the Electrochemical Society, 161(9), p.F889 - F893, 2014/06
Times Cited Count:21 Percentile:61.31(Electrochemistry)Graft-type anion-conducting electrolyte membranes (AEMs) with imidazolium cations on graft polymers were synthesized through radiation-induced graft polymerization of  -vinylimidazole (NVIm) on poly(ethylene-co-tetrafluoroethylene) (ETFE) films, followed by
-vinylimidazole (NVIm) on poly(ethylene-co-tetrafluoroethylene) (ETFE) films, followed by  -propylation and ion-exchange reactions. The
-propylation and ion-exchange reactions. The  -propylation proceeded quantitatively, whereas the ion-exchange reactions in 1 M KOH at 60
-propylation proceeded quantitatively, whereas the ion-exchange reactions in 1 M KOH at 60 C were accompanied by partial
C were accompanied by partial  -elimination of the imidazolium cations(AEM2), which exhibited an ion-exchange capacity (IEC) of 0.85 mmol g
-elimination of the imidazolium cations(AEM2), which exhibited an ion-exchange capacity (IEC) of 0.85 mmol g and ionic conductivity of 10 mS cm
and ionic conductivity of 10 mS cm . AEM2 showed alkaline stability at 60
. AEM2 showed alkaline stability at 60 C but it gradually degraded at 80
C but it gradually degraded at 80 C for ca. 150 h. The copolymer-type AEM (AEM3) with an IEC of 1.20 mmol g
C for ca. 150 h. The copolymer-type AEM (AEM3) with an IEC of 1.20 mmol g was prepared through the copolymerization of NVIm with styrene on ETFE films, followed by the same
was prepared through the copolymerization of NVIm with styrene on ETFE films, followed by the same  -propylation and ion-exchange reactions. AEM3 was shown higher alkaline durability in 1 M KOH at 80
-propylation and ion-exchange reactions. AEM3 was shown higher alkaline durability in 1 M KOH at 80 C. As a result, it exhibited higher conductivity (
C. As a result, it exhibited higher conductivity ( 10 mS cm
10 mS cm ) for 250 h. Therefore, alkylimidazolium cations in copolymer grafts are a promising anion conducting group for alkaline-durable AEMs. A maximum power density of 75 mW cm
) for 250 h. Therefore, alkylimidazolium cations in copolymer grafts are a promising anion conducting group for alkaline-durable AEMs. A maximum power density of 75 mW cm is obtained for AEM3 in a direct hydrazine hydrate fuel cell.
is obtained for AEM3 in a direct hydrazine hydrate fuel cell.
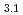 (OCOCH
(OCOCH )
)
 0.9H
0.9H O
OKozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Microporous and Mesoporous Materials, 89(1-3), p.123 - 131, 2006/03
Times Cited Count:11 Percentile:39.24(Chemistry, Applied)Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. Although LTMHSs have recentely attracted attention of researches on anion exchange and intercalation, very limited numbers of reports have been published on their synthesis, characteristics, and applications. This paper describes basic characteristics of a new LTMHS, nickel-copper hydroxide acetate. Hydrothermal Heating of an aqueous solution containing nickel acetate, copper acetate, and hydrogen peroxide to 150 C for 4h yielded a layered compound with an analytical composition of NiCu(OH)
C for 4h yielded a layered compound with an analytical composition of NiCu(OH)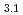 (OCOCH
(OCOCH )
) 0.9H
0.9H O. This compound does not take up Cl
O. This compound does not take up Cl and NO
and NO
 in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
 and AsO
and AsO
 . This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl
. This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl and NO
and NO
 .
.
Kozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Journal of Materials Research, 20(11), p.2997 - 3003, 2005/11
Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. LTMHSs have lately attraced attention of researchs on anion exchange and intercalation but very limited numbers of reports have been published on their synthesis, characteristics, and application. This study reports basic properties of a layered copper hyroxide acetate synthesized by a method modified from that of the previous studies. Titration of copper acetate solution with a dilute NaOH solution to pH 6.5 and subsequent aging at 313 K yielded a layered copper hydroxide acetate. This compound has some properties similar to those of the previously known copper hydroxide acetate, Cu (OH)
(OH) (OCOCH
(OCOCH )H
)H O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
Naganawa, Hirochika; Suzuki, Hideya*; Noro, Junji*; Kimura, Takaumi
Chemical Communications, (23), p.2963 - 2965, 2005/06
A "superweak" anion, TFPB-, gives rise to a field effect on the selectivity for Am over Ln
over Ln in their extraction from aqueous HNO
in their extraction from aqueous HNO solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
Yamaguchi, Tetsuji; Nakayama, Shinichi; Yoshida, Takahiro
Radiochimica Acta, 92(9-11), p.677 - 682, 2004/12
Times Cited Count:6 Percentile:40.63(Chemistry, Inorganic & Nuclear)Adsorption of actinides onto negatively charged mineral surfaces was investigated under conditions that actinides were predominantly present as anionic complex species: Th(CO )
)
 , Am(CO
, Am(CO )
)
 , Np(CO
, Np(CO )
) (OH)
(OH)
 , UO
, UO (OH)
(OH)
 , NpO
, NpO (OH)
(OH)
 , Sn(OH)
, Sn(OH)
 and Pb(OH)
and Pb(OH)
 . These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,
. These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,  -Al
-Al O
O or SiO
or SiO (AEROSIL, specific surface area: 10
(AEROSIL, specific surface area: 10 m
m kg
kg ). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10
). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10 -molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25
-molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25 C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*
Journal of Materials Chemistry, 17(12), p.2993 - 2996, 2002/12
Here we report the extremely high and selective uptake of selenium oxyanions by a novel exchanger, Ni Zn
Zn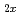 (OH)
(OH) (OCOCH
(OCOCH )
)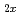 ・nH
・nH O that has brucite-type hydroxide layers with OCOCH
O that has brucite-type hydroxide layers with OCOCH anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10
anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10 ml/g with an initial Se(IV) concentration of 1x10
ml/g with an initial Se(IV) concentration of 1x10 M) in the presence of 0.1M Cl
M) in the presence of 0.1M Cl solution while the well known anionic clay, Mg
solution while the well known anionic clay, Mg Al(OH)
Al(OH) NO
NO ・nH
・nH O showed a Kd of 6.0x10
O showed a Kd of 6.0x10 ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl
ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl , 1N NO
, 1N NO
 , or 1N PO
, or 1N PO
 , while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl
, while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl solution. This novel exchanger also showed high Kd (2.6
solution. This novel exchanger also showed high Kd (2.6 10
10 ml/g at an initial Se concentration of 1
ml/g at an initial Se concentration of 1 10
10 M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
 solutions
solutionsHaba, Hiromitsu; Tsukada, Kazuaki; Asai, Masato; Goto, Shinichi*; Toyoshima, Atsushi; Nishinaka, Ichiro; Akiyama, Kazuhiko; Hirata, Masaru; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.143 - 146, 2002/06
We have investigated the sorption behavior of element 104 rutherfordium (Rf) on an anion exchange resin from HCl and HNO solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl
solutions. In the HCl experiments, the distribution coefficients of Rf increase with an increase of HCl concentration from 7.0 M to 11.5 M, indicating that anionic species such as [Rf(OH)Cl ]
] or [RfCl
or [RfCl ]
] are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO
are formed. This sorption behavior of Rf is typical of the group-4 elements Zr and Hf, and is quite different from that of the pseudo-homologue Th. It is also noted that the distribution coefficients decrease in the order Rf, Zr, Hf at 9.0 M HCl, which is consistent with the expected order of ionic radii. On the other hand, Rf appears to behave like Zr and Hf in 8 M HNO not like Pu and Th.
not like Pu and Th.
 -HI mixed acid solution
-HI mixed acid solutionUsuda, Shigekazu; Sakurai, Satoshi; Hirata, Masaru; Umezawa, Hirokazu
Sep. Sci. Technol., 25(11-12), p.1225 - 1237, 1990/00
Times Cited Count:1 Percentile:27.36(Chemistry, Multidisciplinary)no abstracts in English
Usuda, Shigekazu; Umezawa, Hirokazu
Journal of Radioanalytical and Nuclear Chemistry, Letters, 127(6), p.437 - 446, 1988/06
no abstracts in English
; *; ;
Journal of Radioanalytical and Nuclear Chemistry, Letters, 117(6), p.329 - 336, 1987/06
no abstracts in English
Journal of Radioanalytical and Nuclear Chemistry, 111(2), p.399 - 410, 1987/02
Times Cited Count:15 Percentile:79.68(Chemistry, Analytical)no abstracts in English